 |
||||||||||||
 |
 |
|||||||||||
Electromagnetic Spectrum
Infrared (IR):
The Infrared (IR) radiation is electromagnetic radiation of a wavelength longer than that of visible light, but shorter than that of microwave radiation. The name means "below red" (from the Latin infra, "below"): red being the color of visible light of longest wavelength. The Infrared radiation covers the range from roughly 300 GHz (wavelength of 1 mm) to 400 THz (wavelength of 750 nm). IR is often subdivided into:
- Far-infrared, from 300 GHz (wavelength of 1 mm) to 30 THz (wavelength of 10 μm). The lower part of this range may also be called microwaves. This radiation is typically absorbed by rotational modes in gas-phase molecules, by molecular motions in liquids, and by phonons in solids. The water in the Earth's atmosphere absorbs so strongly in this range that it renders the atmosphere effectively opaque. However, there are certain wavelength ranges ("windows") within the opaque range which allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as "sub-millimeter" in astronomy, reserving far infrared for wavelengths below 200 μm.
- Mid-infrared, from 30 to 120 THz (wavelength of 10 to 2.5 μm). Hot objects (black-body radiators) can radiate strongly in this range. It is absorbed by molecular vibrations: when the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region since the mid-infrared absorption spectrum of a compound is very specific for that compound.
- Near-infrared, from 120 to 400 THz (wavelength of 2500 to 750 nm). Physical processes that are relevant for this range are similar to those for visible light.
The Earth's surface absorbs visible radiation from the sun and re-emits much of the energy as infrared back to the atmosphere. Certain gases in the atmosphere, chiefly water vapor, but also carbon dioxide, methane, nitrous oxide, sulfur hexafluoride, and chlorofluorocarbons, absorb this infrared, and re-radiate it in all directions including back to Earth. Thus, the greenhouse effect keeps the atmosphere and surface much warmer than if the infrared absorbers were absent from the atmosphere. Infrared is used in night-vision equipment, when there is insufficient visible light to see an object. Infrared radiation is also used in Infrared saunas to heat the sauna's occupants and to remove ice from the wings of aircraft (de-icing). Furthermore, Infrared lasers are used to provide the light for optical fiber communications systems Optical telecommunication in the near infrared is technically often separated to different frequency bands because of availability of light sources, transmitting /absorbing materials (fibers) and detectors.: O-band (1260–1360 nm), E-band (1360–1460 nm), S-band (1460–1530 nm), C-band (1530–1565 nm), L-band (1565–1625 nm), and U-band (1625–1675 nm).
Visible Light:
Visible light is the portion of the optical spectrum (light or electromagnetic spectrum) that is visible to the human eye. There are no exact bounds to the optical spectrum, but there are to the visible spectrum. A typical human eye will respond to wavelengths from 400 to 700 nm, although some people may be able to perceive wavelengths from 380 to 780 nm. A light-adapted eye typically has its maximum sensitivity at around 555 nm, in the green region of the optical spectrum. Wavelengths visible to the eye also pass through the "visible window", the region of the electromagnetic spectrum which passes largely un-attenuated through the Earth's atmosphere (although blue light is scattered more than red light, which is the reason the sky is blue). The response of the human eye is defined by subjective testing, but the atmospheric windows are defined by physical measurement.
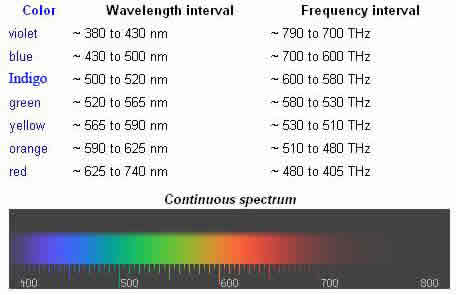 The "visible window" is so called because it overlaps the human visible response spectrum. In addition, the near-infrared (NIR) windows lie just out of human response window. But, the Medium-IR (MIR) and Far-Infrared (FIR) are far beyond the human response region. The eyes of many species perceive wavelengths different than the spectrum visible to the human eye. For example, many insects, such as bees, can see light in the ultraviolet, which is useful for finding nectar in flowers. Visible light radiation is divided into seven different colors (red, orange, yellow, green, blue, Indigo, and violet) that are commonly referred to by their acronym name: Richard Of York Gave Battle In Vain (ROY G. BIV). These colors come from the different energies and frequencies visible light exists in. Red light has a lower frequency while Violet light has the highest frequency. The wavelengths are about 400 nm (violet light) to 700 nm (red light). A red object appears so because it absorbs all the high radiation (colors) and only reflecting the low frequency (red light) to the viewing piece (the eyes).
The "visible window" is so called because it overlaps the human visible response spectrum. In addition, the near-infrared (NIR) windows lie just out of human response window. But, the Medium-IR (MIR) and Far-Infrared (FIR) are far beyond the human response region. The eyes of many species perceive wavelengths different than the spectrum visible to the human eye. For example, many insects, such as bees, can see light in the ultraviolet, which is useful for finding nectar in flowers. Visible light radiation is divided into seven different colors (red, orange, yellow, green, blue, Indigo, and violet) that are commonly referred to by their acronym name: Richard Of York Gave Battle In Vain (ROY G. BIV). These colors come from the different energies and frequencies visible light exists in. Red light has a lower frequency while Violet light has the highest frequency. The wavelengths are about 400 nm (violet light) to 700 nm (red light). A red object appears so because it absorbs all the high radiation (colors) and only reflecting the low frequency (red light) to the viewing piece (the eyes).
Ultraviolet (UV):
Ultraviolet (UV) radiation is electromagnetic radiation of a wavelength shorter than that of the visible region, but longer than that of soft X-rays. The name means "beyond violet" (from Latin ultra, "beyond"), violet being the color of the shortest wavelengths of visible light. Some of the UV wavelengths are colloquially called black light, as it is invisible to the human eye. It can be subdivided into near UV (380 – 200 nm wavelength), far or vacuum UV (FUV or VUV) (200 – 10 nm wavelength), and extreme UV (EUV or XUV) (1 – 31 nm wavelength). When considering the effect of UV radiation on human health and the environment, the range of UV wavelengths is often subdivided into UVA (380 – 315 nm wavelength) UVA is also called as Long Wave or "black light.” UVB (315–280 nm wavelength) is also called as Medium Wave. And, UVC (< 280 nm wavelength) is also called as Short Wave or "germicidal.” In photolithography or in laser technology, the term deep ultraviolet or DUV refers to wavelengths below 300nm wavelength. The Sun emits ultraviolet radiation in the UVA, UVB, and UVC bands, but because of absorption in the atmosphere's ozone layer, 99% of the ultraviolet radiation that reaches the Earth's surface is UVA: some of the UVC light is responsible for the generation of the ozone. Being very energetic, UV can break chemical bonds, make molecules unusually reactive or ionize them, in general changing their mutual behavior: for example, sunburn is caused by the disruptive effects of UV radiation on skin cells that can even cause skin cancer if the radiation damages the complex DNA molecules in the cells (UV radiation is a proven mutagen): see complete list on picture.
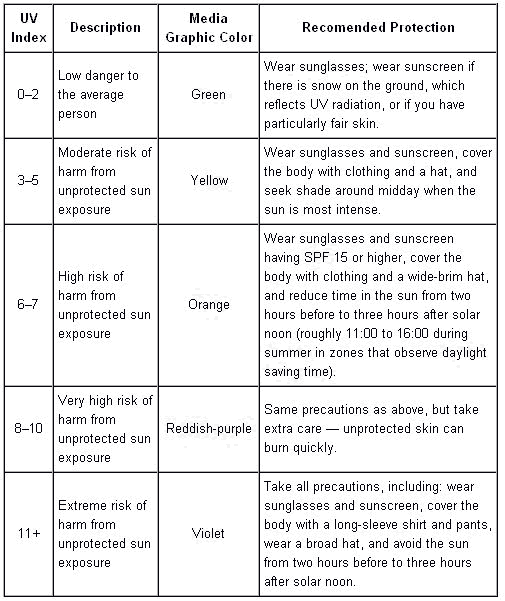
Furthermore, the Sun emits a large amount of UV radiation that could quickly turn Earth into a barren desert. But, most of it is absorbed by the atmosphere's ozone layer before reaching the surface. These waves have very high energy and very short wave lengths: shorter than visible light. Some animals like honey bees can see ultra-violet light. Some plants have white flowers, they are all white to human eyes, but they may appear to be different colors to a honey bee because of the amounts of ultra-violet light which they reflect. We make use of the UV in many ways such as fluorescent lamps, pest control, spectrophotometer, astronomy, sterilization, disinfecting drinking water, fire detector, and etc.
X-Ray or Röntgen ray:
An X-ray or Röntgen ray is a form of electromagnetic radiation with a wavelength in the range of 10 nm to 100 pm (corresponding to frequencies in the range 30 PHz to 3 EHz). X-rays are primarily used for diagnostic medical imaging and crystallography. X-rays are a form of ionizing radiation and can be dangerous. X-rays with a wavelength approximately longer than 0.1 nm are called soft X-rays. At wavelengths shorter than this, they are called hard X-rays. Hard X-rays overlap the range of long wavelength (lower energy) gamma rays; however, the distinction between the two terms depends on the source of the radiation, not its wavelength. In X-ray, photons are generated by energetic electron processes, but photons are generated by transitions within atomic nuclei in gamma rays. The basic production of X-rays is by accelerating electrons in order to collide with a metal target (tungsten usually). Here the electrons suddenly decelerate upon colliding with the metal target and if enough energy is contained within the electron it is able to knock out an electron from the inner shell of the metal atom. As a result electrons from higher energy levels then fill up the vacancy, and X-ray photons are emitted. The detection of X-rays is based on various methods. The most commonly known method is a photographic plate and a fluorescent screen. The X-ray photographic plate is frequently used in hospitals to produce images of the internal organs and bones of a patient. Another method of detecting X-rays is a fluorescent plate. In modern hospitals, a special plastic sheet is used in place of the photographic plate. The plastic sheet is read by a scanning laser beam. The resultant image is then stored in a computer. The plastic sheet can be used over and over again. X-Rays have so much energy and such a short wavelength that they can go right through human body. However, they cannot get through bone as easily as they can get through muscle: this is because bones contain so much Calcium. X-Rays is also used in high-energy physics and astronomy. In the 1990s the Chandra X-Ray Observatory was launched, allowing the exploration of the very violent processes in the universe which produce X-Rays. Unlike visible light, which is a relatively stable view of the universe, the X-ray universe is unstable. It features stars being torn apart by black holes, galactic collisions, and novas, neutron stars that build up layers of plasma that then explode into space.
Gamma Rays:
Gamma rays (often denoted by the Greek letter gamma, γ) are an energetic form of electromagnetic radiation produced by radioactivity or other nuclear or subatomic processes such as electron-positron annihilation. Gamma rays form the highest-energy end of the electromagnetic spectrum. They are often defined to begin at energy of 124 keV, a frequency of 3 EHz, or a wavelength of 10 pm. Although electromagnetic radiation from around 124 keV to several hundred keV is also referred to as hard X rays: there is no physical difference between gamma rays and X rays of the same energy. They are two names for the same electromagnetic radiation: like sunlight and moonlight are two names for visible light. Gamma ray is a term for high-energy electromagnetic radiation produced by nuclear transitions, while X ray is a term for high-energy electromagnetic radiation produced by energy transitions due to accelerating electrons. Because it is possible for some electron transitions to be of higher energy than some nuclear transitions, there is an overlap between what we call low energy gamma rays and high energy X-rays. Gamma rays are a form of ionizing radiation, and they are more penetrating than either alpha or beta radiation (neither of which is electromagnetic radiation), but less ionizing. In terms of ionization, gamma radiation interacts with matter via three main processes which are the following:
- Photoelectric Effect: This describes the case in which a gamma photon interacts with and transfers all of its energy to an orbital electron, ejecting that electron from the atom. The kinetic energy of the resulting photoelectron is equal to the energy of the incident gamma photon minus the binding energy of the electron. The photoelectric effect is thought to be the dominant energy transfer mechanism for x-ray and gamma ray photons with energies below 50 keV (thousand electron volts), but it is much less important at higher energies.
- Compton Scattering: This is an interaction in which an incident gamma photon loses enough energy to an orbital electron to cause its ejection, with the remainder of the original photon's energy being emitted as a new, lower energy gamma photon with an emission direction different from that of the incident gamma photon. The probabilities of Compton scatter decreases with increasing photon energy. Compton scattering is thought to be the principal absorption mechanism for gamma rays in the intermediate energy range 100 keV to 10 MeV (Mega electron Volts), an energy spectrum which includes most gamma radiation present in a nuclear explosion. Compton scattering is relatively independent of the atomic number of the absorbing material.
- Pair Production: By interaction in the vicinity of the coulomb force of the nucleus, the energy of the incident photon is spontaneously converted into the mass of an electron-positron pair. An electron is the matter equivalent of a positron; it has the same weight as a positron, but it has a negative charge equal in strength to the positive charge of a positron. Energy in excess of the equivalent rest mass of the two particles (1.02 MeV) appears as the kinetic energy of the pair and the recoil nucleus. The electron of the pair, frequently referred to as the secondary electron, is densely ionizing. The positron has a very short lifetime. It combines within 10-8 seconds with a free electron. The entire mass of these two particles is then converted into two gamma photons of 0.51 MeV energy each.
Gamma rays produce damage similar to that caused by X-rays such as burns, cancer, and genetic mutations. Gamma rays from nuclear fallout would probably cause the largest number of casualties in the event of the use of nuclear weapons in a nuclear war. An effective fallout shelter reduces human exposure at least 1000 times. The powerful natures of gamma rays have made them useful in the sterilizing of medical equipment by killing bacteria. They are also used to kill bacteria and insects in food stuffs, particularly meat and vegetables, to maintain freshness. Gamma rays are also used for diagnostic purposes in nuclear medicine. Several gamma-emitting radioisotopes are used, one of which is technetium-99m. When administered to a patient, a gamma camera can be used to form an image of the radioisotope's distribution by detecting the gamma radiation emitted. Such a technique can be employed to diagnose a wide range of conditions (e.g. spread of cancer to the bones). Gamma rays also are useful to astronomers in the study of high-energy objects or regions and find a use with physicists due to their penetrative ability and their production from radioisotopes.
Cosmic Rays:
Cosmic rays can loosely be defined as radiation consisting of energetic particles originating outside of the Earth. Cosmic rays are composed mainly of ionized nuclei, roughly 90% protons, 9% helium nuclei and most of the rest being made up of heavier nuclei. Electrons, gamma rays, and very high energy neutrinos also make up a much smaller fraction of the cosmic radiation. Though muons are not stable, the relativistic time dilation may allow them to reach Earth at very high energies. The kinetic energies of cosmic ray particles span over fourteen orders of magnitude, with the flux of cosmic rays on the Earth's surface falling approximately as the inverse cube of the energy. The wide variety of particle energies is reflected in the wide variety of sources. Cosmic rays originate from energetic processes on the Sun all the way to the farthest reaches of the visible universe. Cosmic rays can have energies up to 1020 eV. The ionized nuclei that make up cosmic rays are able to travel from their distant sources to the Earth because of the low density of matter in space. Charged nuclei interact strongly with other matter, so when the cosmic rays approach Earth they begin to collide with the nuclei of atmospheric gases. These collisions, in a process known as a shower, result in the production of many pions and kaons, unstable meson particles which quickly decay into muons. Because muons do not interact strongly with the atmosphere and because of the effect of time dilation many of these muons are able to reach the surface of the Earth. Muons are ionizing radiation, and may easily be detected by many types of particle detectors such as bubble chambers or scintillation detectors. If several muons are observed by separated detectors at the same instant it is clear that they must have been produced in the same shower event. They are several types of cosmic radiation which are the following:
- Anomalous cosmic rays (ACRs) are cosmic rays with unexpectedly low energies. They are thought to be created near the edge of our solar system, in the heliosheath, the border region between the heliosphere and the interstellar medium. When electrically neutral atoms are able to enter the heliosheath (being unaffected by its magnetic fields) subsequently become ionized, they are thought to be accelerated into low-energy cosmic rays by the solar wind's termination shock which marks the inner edge of the heliosheath. It is also possible that high energy galactic cosmic rays which hit the shock front of the solar wind near the heliopause might be decelerated, resulting in their transformation into lower-energy anomalous cosmic rays.
- Galactic cosmic rays (GCRs) are the high-energy particles that flow into our solar system from far away in the Galaxy. GCRs are mostly pieces of atoms: protons, electrons, and atomic nuclei which have had all of the surrounding electrons stripped during their high-speed (almost the speed of light) passage through the Galaxy. Cosmic rays provide one of our few direct samples of matter from outside the solar system. Galactic cosmic rays differ in their composition and origin from solar cosmic rays, which are mostly protons and helium nuclei accelerated by solar activity. The mean energies of galactic cosmic rays also are much higher than the energies of solar cosmic rays.
- Solar cosmic rays are cosmic rays that originate from the Sun. Most are made of protons; these rays are relatively low in energy (10-100 keV). The average composition is similar to that of the Sun itself. The name solar cosmic ray itself is a misnomer, but it has stuck. High energy (MeV and above) cosmic rays come mainly from outside the solar system, while the particles in the solar case are energized near the Sun's surface by the action of magnetic fields. The misnomer arose because there is continuity in the energy spectra, i.e. the flux of particles as a function of their energy, because the low energy solar cosmic rays fade more or less smoothly into the galactic ones as one looks at higher and higher energies.
- Greisen-Zatsepin-Kuzmin limit (GZK limit) is a theoretical upper limit on the energy of cosmic rays from distant sources. This limit was computed in 1966 by Kenneth Greisen, Vadem Kuzmin and Georgi Zatsepin, based on interactions predicted between the cosmic ray and the photons of the cosmic microwave background radiation. They predicted that cosmic rays with energies over the threshold energy of 5×1019 eV would interact with cosmic microwave background photons to produce pions. This would continue until their energy fell below the pion production threshold. Therefore, extragalactic cosmic rays with energies greater than this threshold energy should never be observed on Earth.
Summay of Electromagnatic Spectrum:
The Electromagnetic (EM) spectrum is the range of all possible electromagnetic radiation. Electromagnetic radiation can be described in terms of a stream of photons, which are mass less particles each traveling in a wave-like pattern and moving at the speed of light. Each photon contains a certain amount (or bundle) of energy, and all electromagnetic radiation consists of these photons. The only difference between the various types of electromagnetic radiation is the amount of energy found in the photons. Radio waves have photons with low energies, microwaves have a little more energy than radio waves, infrared has still more, then visible, ultraviolet, X-rays, and the most energetic of all gamma-rays. The EM spectrum of an object is the range of electromagnetic radiation that it emits, reflects, or transmits. Nearly all objects in the universe emit, reflect and/or transmit some light. The distribution of this light along the electromagnetic spectrum is determined by the object's composition. Several types of spectra can be distinguished depending upon the nature of the radiation coming from an object:
- If the spectrum is composed primarily of thermal radiation emitted by the object itself, an emission spectrum occurs.
- If the spectrum is composed of background light, parts of which the object transmits and parts of which it absorbs, an absorption spectrum occurs.
Actually, the electromagnetic spectrum can be expressed in terms of energy, wavelength, or frequency: frequency is measured in cycles per second (Hertz), wavelength is measured in meters, and energy is measured in electron volts. Radio waves generally are utilized by antennas of appropriate size, with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via modulation. Television, mobile phones, wireless networking and amateur radio all use radio waves. The waves used for radio communication (and other purposes) are neatly divided up in decades: divided into bands whose wavelengths and frequencies vary over a factor of 10. In wavelength, the bands begin and end on meters times a power of ten. In frequencies, the bands begin and end on 3 times a power of 10 Hertz (Hz) because the speed of light is close to 3x108 m/s. The bands of the radio spectrum are the following: ELF, SLF, ULF, VLF, LF, MF, HF, VHF, UHF, SHF, and EHF. The ELF, SLF, ULF, and VLF bands overlap the audio frequency (AF) spectrum that is approximately 20–20,000 Hz. The SHF and EHF bands are often considered to be not part of the radio spectrum and form their own microwave spectrum. The radio frequency spectrum can be summary as shown below:
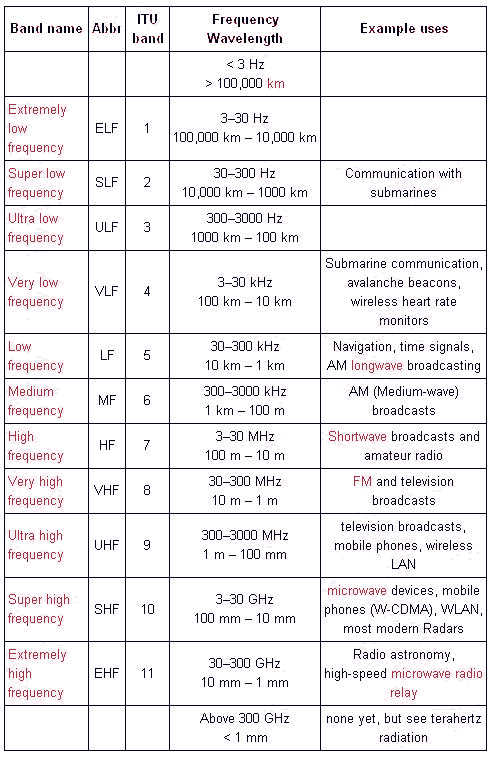
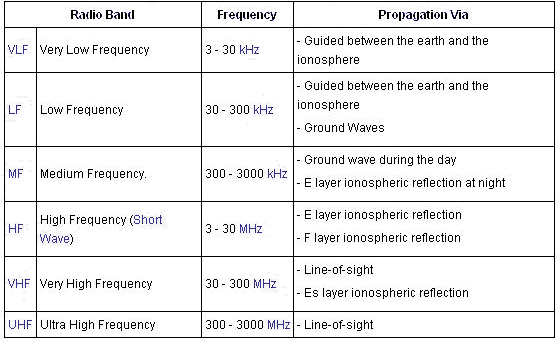
Radio waves at different frequencies propagate in different ways as shown on below:
Furthermore today, radio waves sent at terahertz frequencies, known as terahertz radiation, terahertz waves, T-rays, T-light, T-lux and THz, are in the region of the light spectrum between far infrared and microwaves. Like infrared radiation or microwaves, these waves usually travel in line of sight. Terahertz radiation is non-ionizing and shares with microwaves the capability to penetrate a wide variety of non-conducting materials. They can pass through clothing, paper, cardboard, wood, masonry, plastic and ceramics. They can also penetrate fog and clouds but cannot penetrate metal or water. The Earth's atmosphere is a strong absorber of terahertz radiation, so the range of terahertz radiation is quite short, limiting its usefulness. The proposed WiMAX standard for wireless networking, a long-range enhancement of Wi-Fi, lies within this region. Scientists are also looking to apply Terahertz technology in the armed forces, where high frequency waves will be sent at enemy troops to incapacitate them.
Here ends the radio band. These are just a couple types of electromagnetic radiation which only represents a tiny portion of the whole EM. Hereafter, wavelengths are used almost exclusively, partly for traditional reasons, and partly because frequencies in the THz range (THz = 1012 Hz) are difficult to measure directly. They can be measured by heterodyning: observing the difference frequencies they make with reference signals. While the classification scheme is generally accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. The most common electromagnetic spectrum, which have higher frequency than radio frequency, are Infrared radiation, Visible light radiation, Ultraviolet radiation, X-ray radiation, Gamma Ray radiation, and cosmic rays radiation, and Cosmic rays radiation. The summary of those EM spectrums can be seen below.
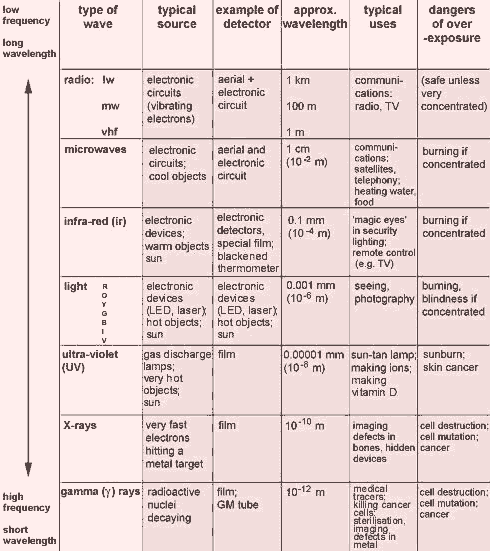
This left us with the Cosmic rays. Cosmic rays can loosely be defined as radiation consisting of energetic particles originating outside of the Earth. Cosmic rays are composed mainly of ionized nuclei, roughly 90% protons, 9% helium nuclei and most of the rest being made up of heavier nuclei. Electrons, gamma rays, and very high energy neutrinos also make up a much smaller fraction of the cosmic radiation. Some of them have ultrahigh energies in the range 100 - 1000 TeV. The origin of cosmic rays is not yet fully known. It is clear, however, that nearly all of them come from outside the solar system, but from within the galaxy. The relatively few particles of solar origin are characterized by temporal association with violent events on the sun and consequently by a rapid variability. In contrast, the bulk of cosmic rays show an autocorrelations with solar activity, being more effectively excluded from the solar neighborhood during periods when the expanding, magnetized plasma from the sun: the solar wind is most intense. Cosmic rays are used by astronomers to gain some insights into the nature and origin of universe.
Click here to go back to Page 1
This site is maintained by Uklit Osatis, it has nothing to do with all company.
It is purely created for researching.
The last update on this web site is on November 27, 2006